96 Well Form
The 96 Well form is a standardized document used primarily in laboratory settings to organize and track sample data. This form allows researchers to efficiently manage multiple samples within a single plate, facilitating accurate data collection and analysis. By utilizing the 96 Well form, laboratories can enhance their workflow and maintain clarity in their experimental processes.
Edit 96 Well Online
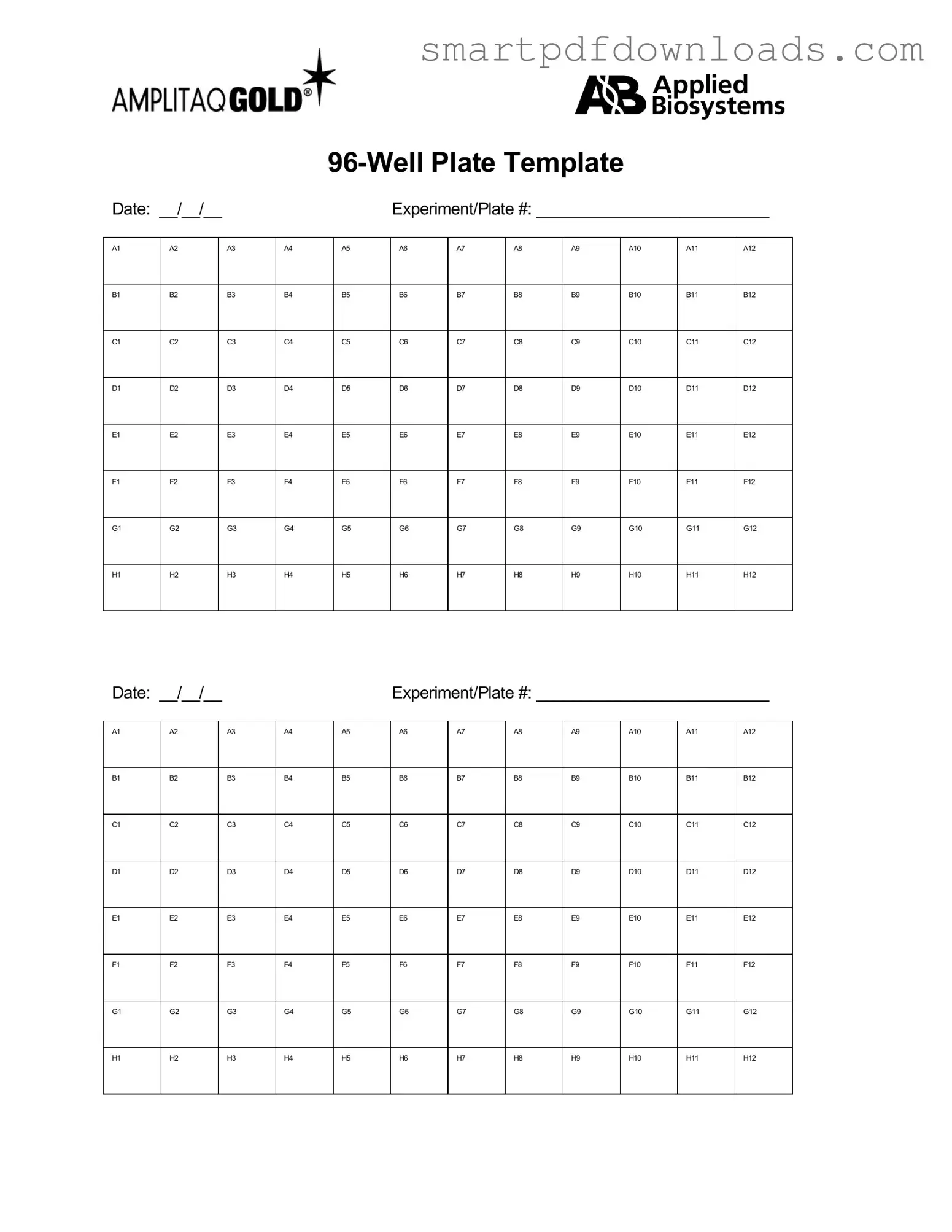
96 Well Form
Edit 96 Well Online

Edit 96 Well Online
or
⇓ PDF File
Finish the form and move on
Edit 96 Well online fast, without printing.
